
Medical engineering requires long-term approach
First there was Brexit, trade wars and structural change in key industries, now it’s the corona pandemic. Up to now the machine tool manufacturers have only been marginally affected by the economic setbacks, but they are now facing increasing threats. Medical engineering readily springs to mind when it comes to markets that still hold growth potential. But is there any point in even considering moving in this direction in the short term? Niklas Kuczaty, Managing Director of the VDMA’s Medical Technology Working Group, is somewhat sceptical: “Medical engineering is a very complex industry. Those looking to gain a foothold need to show commitment and determination – and above all staying power – before any investment will pay off.”
Machine tool manufacturers need to plan carefully before entering this complex Industry
At present, medical engineering represents something of a unicorn in the German industrial landscape. It requires high innovativity and investment levels, but enjoys rising demand that is not dependent on economic cycles, and remains steady and reliable even in times of corona. But where there is light, there is inevitably also shade. Scarcely any other industry is so heavily regulated. The bar has been raised once again in the form of the new European Medical Device Regulation (MDR). Machine tools used for the production of implants and surgical instruments or for micro-milled prosthesis geometries, for example, must offer maximum precision and reliability. Quality assurance plays a decisive role here. When it comes to health, there is no room for compromise. “Anyone looking to enter the medical engineering sector needs to know what they’re getting into,” emphasises Christian Rotsch, Head of the Medical Engineering Department at the Fraunhofer Institute for Machine Tools and Forming Technology (IWU), Dresden/Chemnitz. He regards ISO 9001 certification as a basic requirement. Fraunhofer IWU itself is ISO 9001-certified and it also holds ISO 13485 quality management certification for medical devices.
Focus on manufacturing processes and materials
The Fraunhofer IWU Institute is involved in numerous medical engineering projects. Its main emphasis is on manufacturing processes and materials, but also on biomechanics and on translating project results into clinical treatments. Interdisciplinary cooperation is crucial. One of its key areas is the development of technology for cutting, ablating and forming processes in precision and micro manufacturing. In addition, it is carrying out research into bone-like structures which can be produced from cellular structures, for example from metal foam or with the aid of generative manufacturing processes. There are attempts to enhance material properties through solid forming. Additive manufacturing methods are increasingly being deployed to create customised, patient-specific implants. Nevertheless, Rotsch sees no threat here to the wide range of conventional methods: “Machining and the corresponding machine tools will still be needed in the future,” he points out.
The projects on which the Chemnitz scientists are working involve both medium-sized and large-scale machine tool manufacturers. Christian Rotsch sees very good prospects for SMEs to enjoy success with special solutions and machines, for example in the field of microprocessing and finishing. Complete process chains are also in demand, especially involving robotic support, which Rotsch sees as a “lucrative field with great potential in terms of demand”.
Maximum quality through reliable processes

Combined-process automation cell for medical technology: Exeron’s manufacturing combination including milling, die-sinking EDM, cleaning and measurement, incorporating Erowa zero point clamping system and Certa Systems process control system. Photo: Exeron
One such example is Exeron, based in Oberndorf am Neckar – a system supplier of die-sinking EDM and high-speed milling machines. It joined forces with Erowa (Büren, Switzerland) and Certa Systems (Nuremberg, Germany), to develop a combined-process automation cell for Aesculap (Tuttlingen, Germany), a subsidiary of the B. Braun Group and manufacturer of surgical, orthopaedic and interventional vascular medical products. Aesculap’s challenge is that the required component geometries are sometimes so small, complex and angled that they can no longer be milled and have to be sink eroded instead. In addition, manual retooling processes always pose the risk of inaccuracies creeping in. The desired accuracy was achieved through Exeron’s manufacturing combination of milling, die-sinking EDM, cleaning and measuring; Erowa’s zero-point clamping system which increases precision and speed; and Certa Systems’ process control system which provides automation within the manufacturing network.
According to Udo Baur, Sales Manager for Germany and Europe at Exeron, it is crucial to adapt to the special needs of this sensitive industry and also to be prepared to offer unusual solutions or other special services. This includes providing support for product releases. The automation cell was initially commissioned at Exeron but not handed over to Aesculap until after the product release. “We know our customers and their stringent demands,” says Baur, “and we have the know-how and the machines to meet these.”
Special requirements require tailor-made solutions

Horn's JET-whirling process – developed for the production of high-precision, dimensionally stable bone screws made of titanium and stainless steel – extends tool life and prevents chip accumulation. Photo: Paul Horn
Christian Thiele, Press Spokesman at Hartmetall-Werkzeugfabrik Paul Horn, also reports on the highly specialised requirements for materials, machining systems and tool solutions. “Experience from other industries is only transferable to a certain extent,” he says. Horn is active in specific fields, offering specialised and unique tool solutions in the whirling of bone screws, for example. The precision tool manufacturer was able to significantly increase tool life by internally cooling the whirling tool while simultaneously preventing the risk of chip accumulation. Whirling is used in medical engineering to produce high-precision and dimensionally stable bone screws made of titanium and stainless steel. Horn also offers special solutions for surgical instrument machining in the form of specially ground milling tools or special milling cutters with a high milling depth and very narrow cutting width for surgical forceps. The company is also conducting research into cutter coating solutions for medical engineering materials and into cutting conditions for medical products. Christian Thiele also emphasises the great importance of quality management, which is essential for the manufacture of sophisticated medical products.
Costs for medical devices continuing to rise
IWU expert Christian Rotsch fears that the cost of new medical engineering products will increase dramatically in the future. The Medical Device Regulation requirements are a source of increasing economic pressure for the manufacturers of medical devices, which they are passing on to machine manufacturers and suppliers. Nevertheless, Rotsch is convinced that it will remain worthwhile for machine tool manufacturers and suppliers to enter the medical engineering market. He believes that, if the post-processing stage can be automated, additive processes will give rise to new ideas through the integration of new functions and the trend away from mass-produced towards customised products. However, the most important success factor for companies always remains their response to the regulatory aspects and quality assurance.
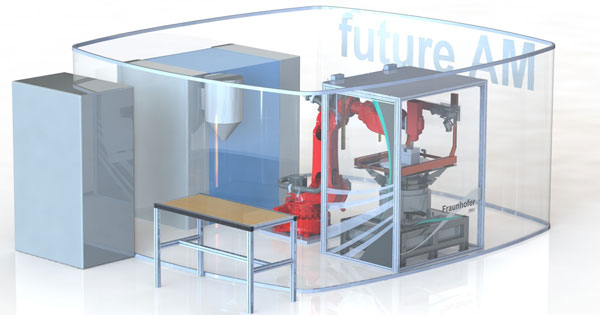
Additive manufacturing processes offer great potential for medical technology, especially in cases where the post-processing stage can be automated. The picture shows an autonomous, robot-supported post-processing cell from the Fraunhofer companies’ Future AM project for the removal of support structures and the creation of target geometries. Photo: Fraunhofer IWU
Paul Horn has been involved in medical engineering for many years, and the sector’s share of total sales is likely to increase in the future. Automotive and medical engineering have traditionally been the strongest pillars of the company, but medical engineering is currently proving to be a stable market for the metalworking company. The corona crisis has generated a number of short-term and urgent enquiries, such as the recent example of a customer who approached Paul Horn with machining problems in the production of components for a heart-lung machine. Paul Horn is maintaining operations in all areas, which will allow it to react quickly and effectively. However, it’s important to realise, says Thiele, that there is no decline in the demand for tools used in the manufacture of implants and hip joints, for example; they are simply out of the limelight at the moment.
VDMA expert Niklas Kuczaty is also convinced of the strong growth prospects for medical engineering, even if they are not expected to reach the volume of the automotive industry. Furthermore, the sector is much less dependent on economic cycles. In any case, companies that decide to enter the market have to realise that they will be investing for at least two to three years before seeing any success. Yet, as Kuczaty points out, taking such a long-term approach will eventually pay off. Maybe not immediately, but perhaps in time for the next crisis.






